Abstract
VITT is an immune-based complication of adenoviral-based vaccines used to immunize against SARS_CoV2. The antibodies in VITT have been described as directed at the platelet-specific chemokine PF4 (CXCL4). While the clinical course and target chemokine in VITT has much in common with the better-known thrombocytopenic/prothrombotic disorder, heparin-induced thrombocytopenia (HIT), which involves antibodies directed against PF4 bound to the polyanion heparin, the specific loci where VITT and PF4/polyanion HIT antibodies bind appear to differ in studies using alanine-scanning mutations of PF4 (Nature, 2021. DOI: 10.1038/s41586-021-03744-4). The VITT antigenic site localizes to a heparin-binding domain. Unlike the dominant HIT locus, the VITT locus is conserved not only between human and mouse PF4, but also between PF4 and the related platelet-specific chemokine NAP2 (CXCL7). NAP2 is also expressed and stored in platelet alpha-granules and is present in equimolar concentrations to PF4. Unlike PF4, NAP2 avidly binds the chemokine receptor CXCR2 and strongly activates neutrophils.
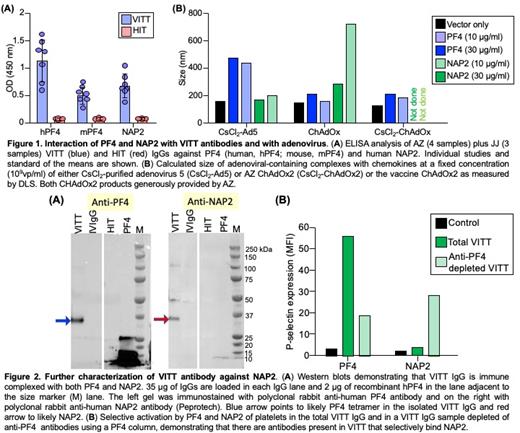
We now show that antibodies from patients who developed VITT after both AstraZeneca (AZ) or Johnson and Johnson (JJ) adenoviral vaccines, unlike HIT antibodies, recognize mouse PF4 (Figure 1A). More importantly, both AZ and JJ VITT antibodies bound NAP2, while none of the HIT antibodies tested bound PF4 or NAP2 in the absence of heparin (Figure 1A). These results are consistent with the alanine-scanning studies that distinguish the HIT and VITT binding sites. Dynamic light scattering (DLS) showed that NAP2 and PF4 bind to the adenoviral vectors, including Ad5 and the AZ vector ChAdOx5, which leads to expression of SARS_CoV2 spike protein. ChAdOx2 vaccine and CsCl 2-purified ChAdOx2 bound to both proteins, but form larger complexes with NAP2 than with PF4 even at lower concentrations of this chemokine (Figure 1C). Removal of anti-PF4 antibodies by hPF4-Sepharose abrogated PF4-dependent binding, but did not significantly reduce binding to NAP2 (not shown), indicating that VITT plasma contains discrete pools of anti-PF4 and anti-NAP2 antibodies that may have distinct functional properties. Sandwich ELISA (not shown) and Western blot analysis of purified VITT IgG demonstrates the presence of hPF4-IgG and NAP2-IgG immune complexes in purified patient's IgG (Figure 2A). Functional studies show that both PF4 and NAP2 can activate platelets in the presence of VITT antibodies. Anti-PF4-depleted VITT IgG fraction retains the ability to activate platelets in the presence of NAP2 (Figure 2B).
Thus, unlike HIT, VITT appears to target a shared antigenic site on the related chemokines PF4 and NAP2. This raises the question as to whether NAP2, as one the most abundant platelet chemokines released from activated platelets, is involved in the initiation and propagation of the immunothrombotic response. Additional studies are needed to see whether NAP2, which can potently and specifically activate neutrophils via CXCLR2, contributes to the specific thromboinflammatory phenotype seen in VITT. We propose using FcgammaRIIA+ mice that concurrently express human PF4 and NAP2 and specific knockout of each chemokine, available in our group, to further understand the pathogenesis of VITT and its thrombocytopenic/ prothrombotic phenotype.
Padmanabhan: Veralox Therapeutics: Membership on an entity's Board of Directors or advisory committees. Cines: Dova: Consultancy; Rigel: Consultancy; Treeline: Consultancy; Arch Oncol: Consultancy; Jannsen: Consultancy; Taventa: Consultancy; Principia: Other: Data Safety Monitoring Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal